First off, firefighters don't seal rooms to put out fires. When you reopen that room where the fire has burned down to embers due to oxygen deprivation, you'd probably have a flashover.
A flashover or “full-room involvement” is the leading cause of firefighter
injuries and deaths. However, sometimes we forget to train on the basics of fire behavior, specifically, remembering when, where, why, and how a flashover will occur.
I’ve heard firefighters state that a flashover is “the rapid fire development followed by full-room
involvement and finally thermal collapse,” and “the sudden full-room involvement in flame.” These definitions are good to know. Remember that flashover is a
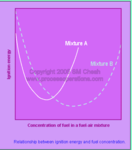
“heat-driven phenomenon.” It’s that simple. If the phenomenon is heat driven, it must be your primary and only concern that you constantly monitor and recognize conditions.
Although the obvious signs of a flashover are
superheated, uninfluenced gases and heat, there are many other factors that will significantly increase your chance of being caught in one. One of the most prevalent factors that influences impending flashover is building construction with illegal modifications. These include, most notably, concealed spaces, energy efficient / hurricane windows, room size and/or ceiling height, and illegal partitions inside occupancies. You must be aware of these components of building construction. Some of these factors might be known prior to entering the building. However, you must discover others if heat levels continue to rise regardless if cooling or venting has taken place.
Other factors also influence a flashover way before the fire starts. For example, cell phones have aided in ensuring we are arriving faster to the fire scene than we did 15 years ago. Almost everyone has and carries a cell phone and knows how to dial 911 when there is an emergency, specifically a fire. We are being made aware of fires faster, especially those in their incipient phases. In return, we are getting on the trucks and to the scene faster than those who did this job 10 to 15 years ago. That being said, you must not allow your adrenalin to take over your ability to perform your job correctly on arrival.
When the bells go off, your natural instincts are to race to the truck, rush getting dressed, and rush to the scene. These factors, once added up, create the potential to make a bad decision or miss a key sign the building may be telling you because you rushed. Unfortunately, the lack of
live fire training coupled with fewer fires has altered the amount of experience the newer generation firefighter receives. The fire service becomes complacent because it is not getting the repetition of fires and experience. However, do not use this concern as an excuse for why you are losing firefighters each year to flashovers.
Today’s modern turn out gear allows you to push deeper and farther than ever before. This amazing technology is ultimately a double-edged sword; it acts as a protecting bubble, providing a false sense of security to the firefighter wearing the gear. The vapor, water, and thermal barriers of most bunker gear do protect you but only for a limited time when things go wrong.
According to IFTSA
Firefighter Principles & Tactics, the bunker gear of today will protect an individual for no more than 15 seconds if succumbed to flashover conditions. Yet, we tend to ignore this fact. More often than not, we find ourselves deeper inside buildings and increasing our chances of being involved in fatal flashover conditions.
Today, fires are much more dangerous than 30 to 40 years ago. The synthetics, plastics, and other “methy-ethel bad stuff” being used to build the furniture and products that typically combust are burning hotter and faster, thereby increasing the intensity and the speed for which the fire travels through the stages.
Now that you know the definition of a flashover, what causes it, and what are the factors that
influence it? Start by developing a plan to avoid it. To be successful, you must not be blind to the signs of an impending flashover. To me, being blind (or stubborn) typically involves your will to “keep pushing” until you find the fire and ultimately extinguish it. This is not your fault; it’s simply the fabric from which you are cut when you swore the oath to becoming a firefighter. We all want to be the first one on scene and the first one to stretch the line into the fire and extinguish it. It’s these “bragging rights” that define us as firefighters. We want to succeed, and succeeding means getting to the fire and putting it out.
Early on in my
career, I always believed fire was the danger, and that fire would be the single most dangerous element I faced. I have since changed this thought process. I have learned over time and through experience that the true danger is not being able to find the fire when inside a structure because of zero visibility. I’ve learned how important reading smoke and identifying conditions are prior to entering a building. These conditions will forecast what my crews and I will face once inside.
An example of why zero visibility and high heat without visible fire is dangerous would be when you and your
crew advance toward where you believe is the location of the fire. If heat levels are doubling and tripling for every 10 to 15 feet, advance the hoseline, and you cannot find the fire while you are being pushed to the floor because of the incredible amount of heat. Alarm bells should be ringing that a flashover is imminent. If not corrected quickly, this is a recipe for disaster and could result in serious injury or a line-of-duty death.
Years ago, many of us were taught to never apply water to smoke. Today, we have completely changed our tune on that outdated tactic. The above scenario clearly dictates that the situation has to be cooled, specifically, overhead. If water does not “rain” down on you after hitting the ceiling (or what is above), then you know the temperature above is well above 1,000 F°.
So, how do you limit and survive a flashover? First, you must remember that not every fire progresses to a flashover, but every fire can. Second, you must not get tunnel vision and miss the signs of flashover. Communication between interior crews is paramount, and you must always have an exit strategy. Regardless, if you are inside the building 10 feet or 100 feet, have a plan and consistently communicate that plan to each other. Search and attack areas must be limited, and interior crews have to be cognizant of how far they have advanced. Use a search rope and be part of everyday search tactics, especially in large occupancies or commercial structures. Finally, always be prepared to “dive or die.” Surviving a flashover means you have approximately 15 seconds of bunker gear protection. Realistically, used gear that has been exposed to heat and elements offers closer to six to eight seconds once you have realized the room is about to flash and your self-contained breathing apparatus (SCBA) mask is still intact.
Limiting your chances for encountering flashover relies on three important factors. First, know and sense the flashover signs and notice the increased heat levels with a thermal imaging camera or your senses. Second, understand the heat-driven phenomenon and the fact that this heat has to be cooled, and quickly. Third, if cooled, the fire needs to be ventilated. Finally, once you have attempted ventilation or cooling and conditions are not changing, put your pride aside and vacate the building. Vacating does not mean you are “going defensive”; it just means you are figuring out what is going on, potentially outside of your view and control. “Regrouping” is a better term that could reveal where the fire is and where another tactic is needed to successfully and safely put out the fire. Your job is to confine and extinguish. If you cannot make this happen and conditions deteriorate, take a step back, reevaluate your tactics, and possibly go another route.
Remember, anyone inside a fire building not wearing a SCBA is most likely dead, so exercise an aggressive risk vs. reward assessment. A successful tour at the firehouse is when everyone goes home to his family. To accomplish that, be aggressive, chose the right nozzle and line for the job, aggressively sweep the floor and ceiling during advancement, perform vigorous nozzle work, and communicate so the attack and ventilation is coordinated. When you have constantly monitored smoke and heat conditions, aggressively applied the right amount of water, and reduced the chances of flashover, your training and experiences have kept you out of harm’s way for yet another tour. This will keep you alive and give you one more chance to leave the firehouse and go home safe!
See:
https://www.fireengineering.com/firefighting/understanding-and-avoiding-a-flashover/
Remember, on Jan. 27, 1967, a fire erupted inside the Apollo command module during a preflight rehearsal test, killing three astronauts who were trapped inside. Astronauts Virgil Grissom, Edward White and Roger Chaffee lost their lives when a fire swept through the command module, or CM.
National Aeronautics and Space Administration (NASA) had launched 16 manned space flights in its Mercury and Gemini programs without a single casualty. “Success had become almost routine for us,” NASA flight director Gene Kranz wrote in his book “Failure Is Not an Option.” “The country had gotten complacent.” Perhaps NASA had gotten complacent as well. In spite of orders from Joseph Shea, Apollo Program Manager, the flammable materials were never removed from the Apollo 1 command module.
With 25 days left before the scheduled launch, the crew of Apollo 1 climbed out of a NASA van into sparkling Florida sunshine on January 27, 1967, and ascended the tower of launch pad 34 for a routine simulated launch test. Clad in their spacesuits and carrying their portable air conditioning packs like office workers toting briefcases, the astronauts crossed the 218-foot-high catwalk with vistas of the blue Atlantic waters washing up on the white beaches of Cape Canaveral before climbing inside their command module perched atop a massive booster rocket.
The “plugs-out” test, during which the module was disconnected from the launch pad’s electrical systems and operating under its own power, was classified as non-hazardous since the rocket was unfueled. To make the countdown rehearsal as realistic as possible, the launch pad team sealed the hatch after the astronauts were strapped into their seats inside
a cabin pressurized with pure oxygen.
Throughout the afternoon, minor glitches and communication issues between the spacecraft and Mission Control in Houston caused repeated delays. Hours behind schedule, early evening darkness settled around the launch pad as the simulated countdown reached a hold with 10 minutes left as attempts continued to resolve the radio issues. “How are we gonna get to the moon if we can’t talk between two or three buildings?” quipped a frustrated Grissom at 6:30 p.m.
Less than a minute later, the engineers watching the capsule cabin on a closed-circuit television screen were startled by a flash. A spark that likely came from faulty electrical wiring behind a panel door below Grissom’s feet suddenly ignited in the capsule. Fed by the cabin’s pure oxygen, the spark took only seconds to morph into an inferno that tore through the flammable nylon netting and Velcro surrounding the astronauts.
“Hey! We’ve got a fire in the cockpit!” yelled one of the astronauts. Horrified engineers watched on their screens as smoke filled the cabin while White desperately attempted to release the cumbersome hatch. “We have a bad fire! We’re burning up!” came another screaming transmission from the cockpit.
Then silence.
Pad safety workers grabbed extinguishers and rushed to the capsule, but the dense smoke reduced visibility to nearly zero. Even rescuers wearing smoke masks were overcome by toxic fumes, and the tremendous heat burned through their gloves.
It took more than five minutes for the pad crew to open the complicated latch system on the hatch. By that point, it was far too late. The astronauts had virtually no time to unstrap themselves from their seats, let alone escape the flash fire. Burning at hotter than 1,000 degrees Fahrenheit, the blaze melted the astronauts’ space suits and oxygen tubes. The crew likely lost consciousness and died from asphyxiation from inhaling toxic gases. The process of removing the men from the charred capsule couldn’t begin until six hours after the fire, and it took 90 minutes to extricate their bodies, which were fused to the nylon of the cabin interior.
“We did not do our job! We were rolling the dice, hoping that things would come together by launch day, when in our hearts we knew it would take a miracle,” an emotional Kranz told his flight control team three days after the tragedy. “We were too ‘gung-ho’ about the schedule, and we blocked out all of the problems we saw each day in our work. Every element of the program was in trouble, and so were we.”
NASA indeed pressed ahead with the Apollo program, but more than 20 months elapsed before American astronauts returned to the skies. During that time, NASA made thousands of changes to the Apollo spacecraft, including redesigning the hatch, altering the cabin atmosphere to include nitrogen and replacing flammable materials from the interior.
“It was perhaps the defining moment in our race to get to the moon,” Kranz wrote of the fire aboard Apollo 1. “The ultimate success of Apollo was made possible by the sacrifices of Grissom, White and Chaffee. The accident profoundly affected everyone in the program. There was an unspoken promise on everyone’s part to the three astronauts that their deaths would not be in vain.”
Out of the ashes of the Apollo 1 tragedy came crucial safety and performance improvements that allowed NASA to fulfill Kennedy’s pledge in July 1969 by landing Neil Armstrong and Edwin “Buzz” Aldrin on the moon and returning them safely to Earth. Before departing the lunar surface, the Apollo 11 astronauts left behind a reminder of their fallen colleagues—a commemorative medallion bearing the names of Grissom, White and Chaffee.
See:
https://www.history.com/news/remembering-the-apollo-1-tragedy
As far human breath rekindling a fire in low oxygen circumstances, that is sort of a non-starter as far as I can see.
If it is in an enclosed space, you risk a flash over by opening a means of ingress into the oxygen deprived space.
In a low oxygen open area, you must remember that humans an only exist within certain oxygen parameters, ca. 19-23% oxygen.
As a result, I'm not sure in what scenario that might require human intervention where breath would be required to rekindle a fire. In a fireplace you use bellows to force atmospheric air into a fire quelled to embers to reignite it. Blowing into a fireplace is inherently dangerous including burns, inhaling soot, making a mess.
Note the above, a pure oxygen atmosphere is highly dangerous while a low oxygen atmosphere is equally hazardous.
Hartmann352