In May 2020 Cancer researchers from the University of Texas set out in some detail why Gileads drug GS-441524 was superior to Gileads Remdesivir as a Coronavirus treatment. This was based both on animals trials and previous research on GS-441524. In some animals trials GS-441524 was compared directly with Remdesivir, as an anti coronavirus treatment, with GS-441524 becoming the preferred treatment.
Gilead developed Remdesivir with the FDA, however it is much more complicated and expensive to make than GS-441524 and could be less effective but for numerous reasons Gilead will make more money from Remdesivir and the FDA may also has a financial interest in remdesivir.
Critics say a long known promising Covid-19 treatment GS-441524 has not been studied and that it was delibrately blocked, for profit reasons, from Covid-19 researchers, having proper access to all the information on it.
Prior lab studies have shown that GS-441524 is effective against coronaviruses SARS, MERS and FIP a near 100% fatal cat coronavirus that GS-441524 had a near 100% cure rate for.
Gileads' responses have not addressed the points raised nor whether Gilead is promoting remdesivir because of its longer patent or why Gilead blocked and/or also had not encouraged or helped anyone researching the medical effectiveness of GS-441524 to resolve matters.
Gilead has for a some years also refused to explain why it has not allowed the manufacture or licensing of GS-441524 for use in cats after successful animal trials cured cats of a fatal coronavirus disease

 www.statnews.com
www.statnews.com
A journalist writing in The Atlantic said "After Ebola trials in previous years found little benefit, remdesivir became a drug in search of a (human) disease."
Some years ago GS-441524 and Remdesivir were both tested against FIP a 95% fatal cat coronavirus.
Gilead was involved in these University of California animal tests providing the 25+ different drugs initially screened for use in the trials and credited in the published research.
The trials showed both remdesivir and GS-441524 were effective against FIP however GS-441524 was preferred and cured 100% of the coronavirus infected cats in the university tests. Later field tests on pet cats confirmed GS-441524s effectiveness
Strangely Gilead has refused to allow anyone to make or legally use GS-441524 on cats (or any animals or humans) despite repeated requests related to cats
Thus long before Covid-19 cat owners were forced to buy GS-441524 on the black market to treat their cats and Gilead did nothing nor provided any explaination
Critics say Gilead blocks GS-441524 as it is easier and cheaper to make and so will compete against Remdesivir meaning Gilead will make less money
Following Gileads and the FDAs focus on remdesivir and their failure to enter into meaningful discussions or research on GS-441524, an open letter was written to the FDA and Gilead by a citizens advocacy group asking
why GS-441524 was not being studied as a Covid-19 treatment and
that the FDA and others be encouraged to do research or
to explain why it was not scientifically and medically possible to do so.
The citizens group writing the letter has formally raised medical and other matters over many years, is non political and has been critical of both the Trump administration and the FDA and their response to covid-19 and other citizens rights matters. For example they separately launched legal action against the FDA over what is said to be an unsafe medical device that the FDA failed to withdraw.
The FDA has just responded to the GS-441524 coronavirus research letter saying that they have now
“reviewed the literature and agree that this compound merits further exploration,”
View: https://twitter.com/i/web/status/1297572580853915648
The questions the seem important are
why has it taken so long to start this work,
why did it take a citizens group not doctors or researchers or health officials to get this to happen and
will others also now be encouraged to also do research or will it remain a limited work within the FDA
will Gilead do all it can to help serious researchers resolve if GS-441524 is more effective than remdesivir
does the FDA have a financial interest in remdesivir which is why it favors that drug
Gilead has yet to indicate that it will allow others outside the FDA to view any data it or the FDA holds or encourage third party research.
Research indicates that GS-441524 is less toxic than remdesivir so can be given in higher doses and is much easier to make than remdesivir plus has many other benefits over remdesivir.
Texas University researchers have responded to questions about why GC-441524 is probably superior - see statnews article already linked from May 2020 and the following q&a posted

 www.statnews.com
www.statnews.com
A private Dr highlighted GS-441524 in Feb 2020 in a Tampa Fl news article.
According to journalists and others Gilead has not responded to requests to clarify its position on allowing GS-441524 for the treatment for cats and seems unwilling to provide any meaningful response to requests for clarification on a range of matters about GS-441524 including encouraging meaningful covid-19 research. Gileads responses have focused on stressing remdesivir benefits or passing comments about any researching of GS-441524
Gilead and the FDA jointly developed the drug Remdesivir however Gilead registered the patent without including any FDA rights though later published research papers clearly list the many government scientists involved

 mbio.asm.org
mbio.asm.org
GS-441524 was also developed and patented with other parties and is a very similar drug to remdesivir. The Gilead patent life for Remdesivir is 7+ years longer than the GS-441524 patent and for other reasons the GS-441524 patent may be less profitable for Gilead.
Gilead has prior history of trying to exclude the FDA from participating in patent royalities the FDA contributed to developing.

 www.statnews.com
www.statnews.com
In a letter to leadership of Gilead Sciences, Inc., the National Institutes of Health, the Food and Drug Administration, and the Biomedical Advanced Research and Development Authority, Public Citizen and two scientists specializing in cancer drug development offer a detailed summary of scientific evidence suggesting that the experimental antiviral drug GS-441524 may be a better drug than the closely-related drug remdesivir to treat COVID-19. The letter asks Gilead and the federal agencies either to work collaboratively to promptly pursue the development of GS-441524 as a treatment for COVID-19 or to publicly explain and provide evidence as to why doing so is not scientifically or medically feasible.
The links to the open letter to Gilead and the FDA and other articles and info are below
Open letter to Gilead and FDA
FDA response
View: https://twitter.com/i/web/status/1297572580853915648
“reviewed the literature and agree that this compound merits further exploration,”
Michael Abrams Phd , health researcher at Public Citizen and lead author of the letter to the FDA states “Such further study of the drug is long overdue, especially given the evidence that GS-441524 may be cheaper and easier to manufacture and more amenable to administration in inhaled or oral forms than remdesivir.”
“We hope that Gilead will respond similarly and commit to working collaboratively with the NIH to study the potential of GS-441524, even if it means the company may reap lower profits than expected from the marketing of remdesivir,” Abrams added.

Research on GS-441524

 www.sciencenews.org
www.sciencenews.org
Lab tests have suggested GS-441524 is active against SARS-CoV-2, and it is apparently similar or superior to the effects of remdesivir at levels that do not cause much toxicity, according to the Anderson researchers, who want to run their own tests. They also maintain the compound is more easily synthesized than remdesivir, so it should be easier to create oral versions and make higher doses.

 www.statnews.com
www.statnews.com
The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies
10 cats were infected with FIP and then treated with GS-441524. All 10 cats recovered.
Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis, CA, USA
Gilead Sciences, Foster City, CA, USA
Accepted 14 April 2018

 www.sciencedirect.com
www.sciencedirect.com
Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses
Susan Amirian
Julie K.Levy
Public Health & Healthcare Program, Texas Policy Lab, School of Social Sciences, Rice University, Houston, TX, USA
Maddie's Shelter Medicine Program, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA
Accepted 24 March 2020,

 www.sciencedirect.com
www.sciencedirect.com
The Atlantic
The background to the long term Cat research GS-441524 and the treatment people are using to cure cats of a deadly coronavirus that kills around 95% of infected cats
Around 5 years ago Gilead sent University researchers 25 or 30 molecules, drawn from the large library of drug candidates that pharmaceutical companies typically maintain.
Two of the molecules worked marvelously in cat cells infected with the FIP virus: GS-441524 and GS-5734, the latter of which is now better known as remdesivir.
After Ebola trials found little benefit, remdesivir became a drug in search of a (human) disease.
Both molecules were effective, so Pedersen decided to pursue the simpler one, GS-441524. He then infected 10 cats with FIP and dosed them with GS-441524. All 10 cats recovered.
“We almost fell out of our chairs,” says Weigner.
in a follow-up field trial of 31 pets with naturally acquired FIP, 25 ultimately made it—an unheard-of recovery rate.
Despite the success, Gilead refused to license GS-441524 for use in cats.
While Pedersen was testing GS-441524 in cats, a different virus—a human virus—was raging halfway around the world in West Africa: Ebola. The virus that causes Ebola is not a coronavirus, but remdesivir is unusually broad-acting for an antiviral, and early results against Ebola were promising. So promising, in fact, that the company was eyeing FDA approval of remdesivir in humans.
According to Pedersen, Gilead worried that the cat research could impede the approval process for remdesivir. Because GS-441524 and remdesivir are so similar, any adverse effects uncovered in cats might have to be reported and investigated to guarantee remdesivir’s safety in humans.
Gilead’s caution about generating unnecessary cat data is standard industry practice. “One of the rules in drug development is never perform a test you don’t have to, if the results could be problematic,” says Richard Sachleben, a retired pharma-industry researcher.
(Gilead declined to comment for this story.)

 www.theatlantic.com
www.theatlantic.com

 www.chicagotribune.com
www.chicagotribune.com
Pharmalot
The U.S. government contributed research to a Gilead remdesivir patent — but didn’t get credit
By Ed Silverman @Pharmalot
May 8, 2020
September 2015 in which Gilead sought a U.S. patent for a using the compound for any number of coronavirus infections. Although the code Gilead assigned to the compound – GS-5734 – does not appear in the body of the application, experts who have reviewed the chemical structure say the compound is remdesivir.
One month later, some of the same Gilead employees whose names appeared on the patent application were listed as co-authors on a Nature research paper – along with numerous government scientists – showing remdesivir, specifically, held promise in fighting Ebola and other coronaviruses. The paper also noted testing was conducted at high-risk security labs run by the federal government.
The role played by the federal government in developing remdesivir to combat coronaviruses has, in fact, involved various grants to universities, as well as contributions from government personnel at such agencies as the U.S. Army Medical Research Institute of Infectious Diseases, according to Knowledge Ecology International, an advocacy group that first disclosed these connections.
But while it remains unclear the extent to which federal funds contributed to the R&D, the patent is of particular interest because it is tangible evidence that government work yielded something of potential financial value to the company. Yet government employees are not listed as inventors, which one expert suggested should be corrected, especially in an era when federally financed research might be leveraged to collect royalties or, arguably, lower the price of medicines.
“In this case, all of these scientists were really working together very intimately on research that led to the molecule,” said Arti Rai, a Duke University law professor who specializes in intellectual property and a former U.S. Patent and Trademark Office official, who is researching remdesivir patents and the role played by the federal government.
“Gilead actually had a huge number of patents on the molecules, but had to do a tremendous amount of work to figure out which variations of the various molecules would work against the biological models the government had. This patent illustrates the essence of why collaboration between the public and private sectors is important, not just in terms of money, such as grants, but resources.”
“What really matters is how much money the government has put into research, but if their names were on there, it would help to make the case there was a lot of in-kind contribution from the government,” she explained. “Right now, if Gilead tried to assert rights (in response to a patent challenge), there would be no way to know there was some government right to a license to the patent.”
Generally, one must play connect the dots to find government assistance. For instance, the National Institutes of Health gave grants to several universities whose researchers worked with Gilead scientists. Their efforts appeared in a 2018 paper in the American Society for Microbiology, which found remdesivir can combat coronaviruses, noted Tahir Amin, who heads the Initiative for Medicines, Access, and Knowledge, an advocacy group that challenged various Gilead patents on hepatitis C medicines.
We asked Gilead, which several months ago sought to add claims to the patent, for comment and will update you accordingly.
Numerous consumer advocates, academics, and lawmakers have argued that prescription drugs invented with taxpayer dollars should be affordable to Americans. Public Citizen estimated that U.S. taxpayers contributed $70.5 million to remdesivir R&D overall.
“Since we’re paying for the research, we shouldn’t expect to be at disadvantage,” said Jamie Love of Knowledge Ecology International. “The argument that you have to have a high price because a company made big investments is harder to justify when there was a large public financing role in the R&D. A high company may say a high price is necessary, but the rationale falls apart when we’re paying for the R&D.”
Gilead has often been at the center of such debates. As noted previously, the drug maker and the federal government filed dueling lawsuits over patents that formed the basis of a best-selling HIV prevention pill called Truvada. The government claims Gilead should pay royalties on its patents, which stemmed, in part, from taxpayer funding, while the company has argued the government patents are invalid.

 www.statnews.com
www.statnews.com
Feb 2020 news article on GS-441524
By Dr. Joette Giovinco
Dr. Joette Giovinco wrote a press article highlighting GS-441524 in Feb 2020 as a promising Covid-19 treatment

 www.fox13news.com
www.fox13news.com
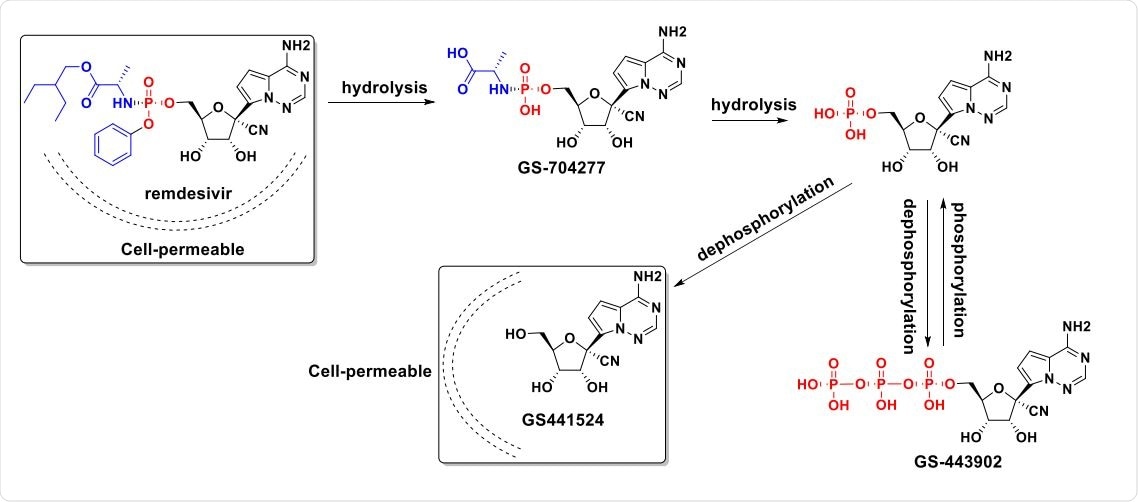
Remdesivir is a promising antiviral drug and works by inhibiting the activity of RNA dependent RNA polymerase (RdRp).
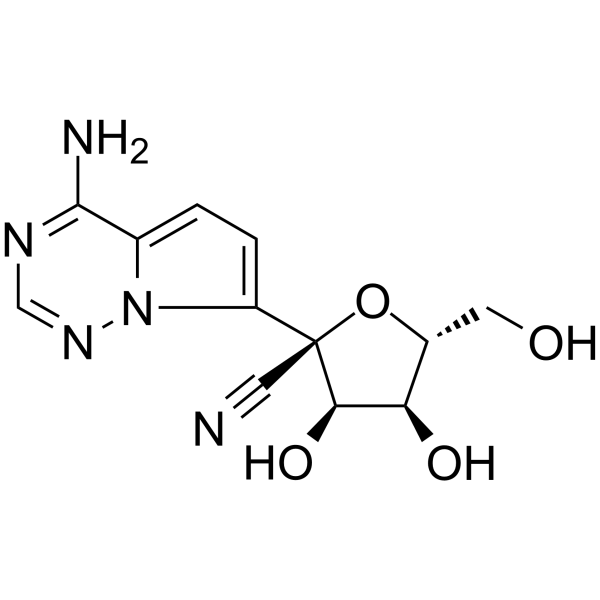
GS-441524 is a feline infectious peritonitis virus (FIPV) inhibitor and a predominant metabolite of Remdesivir that is superior to Remdesivir against COVID-19.
First GS-441524 shows comparable efficacy in cell-based models of primary human lung and cat cells infected with the coronavirus. GS-441524 could strongly inhibit feline infectious peritonitis virus (FIPV), with an EC50of 0.78 μM.
Recent research in coronavirus-infected nonhuman primates demonstrated problems with remdesivir that inadvertently showed the antiviral effectiveness of GS-441524. In multiple studies testing remdesivir in coronavirus-infected mice or rhesus macaques, it was rapidly converted to GS-441524 in the bloodstream..... injection of remdesivir in NHP results in GS-441524 being present in serum at concentrations 1000-fold higher than remdesivir throughout a 7-day treatment course (Figure 3b).
Last but not the least, GS-441524 is capable of treating cats suffering from feline infectious peritonitis (FIP) with a 96% cure rate. Importantly, coupled with the robust antiviral activity of GS-441524 against FIP, these data compel further investigations into the therapeutic and prophylactic utility of GS-441524 against SARS-CoV-2.
All in all, GS-441524, a FIPV inhibitor, is a predominant metabolite of Remdesivir and superior to Remdesivir against COVID-19.

 www.statnews.com
www.statnews.com

 www.livescience.com
www.livescience.com
GILEAD owns patent rights to GS-441524 (2009) and Remdesivir (2017); The 2009 patent of GS-441524 may be invalid due to prior art: as we have seen in the GILEAD vs MERK trial (which Merk initially won), there is a large amount of prior art in the nucleoside space that may invalidate the GS-441524 patent.
 www.patentoppositions.org
www.patentoppositions.org
Covid-19 treatnent research drug GC376
A final thought - great research is being done on Covid-19 some of it is below on another drug also tested on cats with FIP. However this drug GC376 was much less successful than GS-441524
The researchers of GC376 are clearly very good and professional
However how is it Covid-19 treatment research can be done on GC376 with cat coronavirus FIP treatment effectiveness being cited as one of the reasons for rapid research, and yet this not happen on GS-441524 when its almost 100% effective against the cat coronavirus FIP and worked well against other coronaviruses plus GS-441524 is potentially better than remdesivir ?
antiviral GC376 out of Kansas State University had previously been tested but only seven out of 20 cats had gone into remission. Those results seemed impressive at the time, but GS-441524 appeared to be even better
"Relapses no longer responsive to treatment occurred in 13 of these 19 cats within 1-7 weeks of initial or repeat treatment(s). Severe neurologic disease occurred in 8/13 cats that failed treatment and five cats had recurrences of abdominal lesions. At the time of writing, seven cats were in disease remission."

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
Research on GC376

 www6.slac.stanford.edu
www6.slac.stanford.edu
Published: 27 August 2020
Feline coronavirus drug GC376 inhibits the main protease of SARS-CoV-2 and blocks virus replication
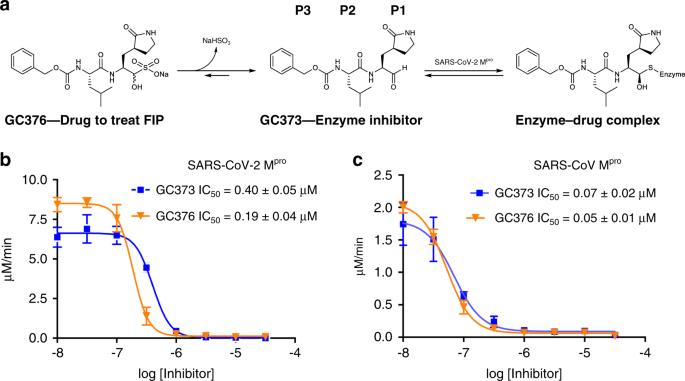
GC376 has been used to successfully treat FIP in cats as its breakdown product, GC373, effectively inhibits the Mpro of FCoV. Analogs of these drugs also inhibited the MERS CoV Mpro and blocked viral replication in cells at an EC50 of 0.5 μM14. Our studies show GC376 and GC373 to be effective inhibitors of SARS-CoV-2 Mpro, in agreement with previous studies showing GC373 and GC376 possess broad specificity against the Mpro of coronaviruses and calciviruses (Supplementary Table 2)10,13,14,22. Clearly, these drugs need to be advanced quickly into human trials for COVID-19. SARS-CoV-2 is the cause of COVID-19 and is a virus with a significant mutation rate23. Also, in some patients the virus has persisted longer than 2 months with some possibility of re-infection24. Vaccines are critically important, but still likely a year or more away as this virus may present vaccine challenges.
There are many clinical trials testing drugs re-purposed from their original indications. Remdesivir, a polymerase inhibitor developed as a treatment for Ebola virus25 had initially been used in compassion cases, and is now FDA approved for emergency use in patients with COVID-19......
 www.nature.com
www.nature.com
Gilead developed Remdesivir with the FDA, however it is much more complicated and expensive to make than GS-441524 and could be less effective but for numerous reasons Gilead will make more money from Remdesivir and the FDA may also has a financial interest in remdesivir.
Critics say a long known promising Covid-19 treatment GS-441524 has not been studied and that it was delibrately blocked, for profit reasons, from Covid-19 researchers, having proper access to all the information on it.
Prior lab studies have shown that GS-441524 is effective against coronaviruses SARS, MERS and FIP a near 100% fatal cat coronavirus that GS-441524 had a near 100% cure rate for.
Gileads' responses have not addressed the points raised nor whether Gilead is promoting remdesivir because of its longer patent or why Gilead blocked and/or also had not encouraged or helped anyone researching the medical effectiveness of GS-441524 to resolve matters.
Gilead has for a some years also refused to explain why it has not allowed the manufacture or licensing of GS-441524 for use in cats after successful animal trials cured cats of a fatal coronavirus disease

Gilead should ditch remdesivir and focus on its simpler and safer ancestor
Gilead has another antiviral that's easier to make and safer to use than remdesivir. Why isn't it giving that drug any attention? The world can only hope it isn't for the sake of protecting its intellectual property.
A journalist writing in The Atlantic said "After Ebola trials in previous years found little benefit, remdesivir became a drug in search of a (human) disease."
Some years ago GS-441524 and Remdesivir were both tested against FIP a 95% fatal cat coronavirus.
Gilead was involved in these University of California animal tests providing the 25+ different drugs initially screened for use in the trials and credited in the published research.
The trials showed both remdesivir and GS-441524 were effective against FIP however GS-441524 was preferred and cured 100% of the coronavirus infected cats in the university tests. Later field tests on pet cats confirmed GS-441524s effectiveness
Strangely Gilead has refused to allow anyone to make or legally use GS-441524 on cats (or any animals or humans) despite repeated requests related to cats
Thus long before Covid-19 cat owners were forced to buy GS-441524 on the black market to treat their cats and Gilead did nothing nor provided any explaination
Critics say Gilead blocks GS-441524 as it is easier and cheaper to make and so will compete against Remdesivir meaning Gilead will make less money
Following Gileads and the FDAs focus on remdesivir and their failure to enter into meaningful discussions or research on GS-441524, an open letter was written to the FDA and Gilead by a citizens advocacy group asking
why GS-441524 was not being studied as a Covid-19 treatment and
that the FDA and others be encouraged to do research or
to explain why it was not scientifically and medically possible to do so.
The citizens group writing the letter has formally raised medical and other matters over many years, is non political and has been critical of both the Trump administration and the FDA and their response to covid-19 and other citizens rights matters. For example they separately launched legal action against the FDA over what is said to be an unsafe medical device that the FDA failed to withdraw.
The FDA has just responded to the GS-441524 coronavirus research letter saying that they have now
“reviewed the literature and agree that this compound merits further exploration,”
View: https://twitter.com/i/web/status/1297572580853915648
The questions the seem important are
why has it taken so long to start this work,
why did it take a citizens group not doctors or researchers or health officials to get this to happen and
will others also now be encouraged to also do research or will it remain a limited work within the FDA
will Gilead do all it can to help serious researchers resolve if GS-441524 is more effective than remdesivir
does the FDA have a financial interest in remdesivir which is why it favors that drug
Gilead has yet to indicate that it will allow others outside the FDA to view any data it or the FDA holds or encourage third party research.
Research indicates that GS-441524 is less toxic than remdesivir so can be given in higher doses and is much easier to make than remdesivir plus has many other benefits over remdesivir.
Texas University researchers have responded to questions about why GC-441524 is probably superior - see statnews article already linked from May 2020 and the following q&a posted

Gilead should ditch remdesivir and focus on its simpler and safer ancestor
Gilead has another antiviral that's easier to make and safer to use than remdesivir. Why isn't it giving that drug any attention? The world can only hope it isn't for the sake of protecting its intellectual property.
A private Dr highlighted GS-441524 in Feb 2020 in a Tampa Fl news article.
According to journalists and others Gilead has not responded to requests to clarify its position on allowing GS-441524 for the treatment for cats and seems unwilling to provide any meaningful response to requests for clarification on a range of matters about GS-441524 including encouraging meaningful covid-19 research. Gileads responses have focused on stressing remdesivir benefits or passing comments about any researching of GS-441524
Gilead and the FDA jointly developed the drug Remdesivir however Gilead registered the patent without including any FDA rights though later published research papers clearly list the many government scientists involved

Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease
Emerging coronaviruses (CoVs) cause severe disease in humans, but no approved therapeutics are available. The CoV nsp14 exoribonuclease (ExoN) has complicated development of antiviral nucleosides due to its proofreading activity. We recently reported that the nucleoside analogue GS-5734...
GS-441524 was also developed and patented with other parties and is a very similar drug to remdesivir. The Gilead patent life for Remdesivir is 7+ years longer than the GS-441524 patent and for other reasons the GS-441524 patent may be less profitable for Gilead.
Gilead has prior history of trying to exclude the FDA from participating in patent royalities the FDA contributed to developing.

The U.S. government contributed research to a Gilead remdesivir patent — but didn't get credit
"This patent illustrates the essence of why collaboration between the public and private sectors is important," said Duke University law professor Arti Rai.
In a letter to leadership of Gilead Sciences, Inc., the National Institutes of Health, the Food and Drug Administration, and the Biomedical Advanced Research and Development Authority, Public Citizen and two scientists specializing in cancer drug development offer a detailed summary of scientific evidence suggesting that the experimental antiviral drug GS-441524 may be a better drug than the closely-related drug remdesivir to treat COVID-19. The letter asks Gilead and the federal agencies either to work collaboratively to promptly pursue the development of GS-441524 as a treatment for COVID-19 or to publicly explain and provide evidence as to why doing so is not scientifically or medically feasible.
The links to the open letter to Gilead and the FDA and other articles and info are below
Open letter to Gilead and FDA

Public Citizen, Scientists: Gilead and Federal Scientists Have Neglected a Potentially Promising COVID-19 Antiviral Drug - Public Citizen
WASHINGTON, D.C. – Gilead Sciences, Inc. and the federal government apparently have been sitting on a potentially promising coronavirus treatment…
www.citizen.org
FDA response
View: https://twitter.com/i/web/status/1297572580853915648
“reviewed the literature and agree that this compound merits further exploration,”
Michael Abrams Phd , health researcher at Public Citizen and lead author of the letter to the FDA states “Such further study of the drug is long overdue, especially given the evidence that GS-441524 may be cheaper and easier to manufacture and more amenable to administration in inhaled or oral forms than remdesivir.”
“We hope that Gilead will respond similarly and commit to working collaboratively with the NIH to study the potential of GS-441524, even if it means the company may reap lower profits than expected from the marketing of remdesivir,” Abrams added.

NIH Agrees With Public Citizen, Will Conduct Preclinical Trials of the Potential COVID-19 Treatment GS-441524 - Public Citizen
WASHINGTON, D.C. – The National Institutes of Health (NIH) will expeditiously conduct preclinical studies of GS-441524 as a treatment for…
www.citizen.org
Research on GS-441524

How two coronavirus drugs for cats might help humans fight COVID-19
Scientists are exploring if drugs for a disease caused by a coronavirus that infects only cats might help also people infected with the coronavirus.
Lab tests have suggested GS-441524 is active against SARS-CoV-2, and it is apparently similar or superior to the effects of remdesivir at levels that do not cause much toxicity, according to the Anderson researchers, who want to run their own tests. They also maintain the compound is more easily synthesized than remdesivir, so it should be easier to create oral versions and make higher doses.

Urged on by scientists, NIH will study Gilead's remdesivir-like compound against Covid-19
The NIH plans to independently explore whether a Gilead compound — which some academics say is highly similar to remdesivir — might be useful in combating Covid-19.
The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies
10 cats were infected with FIP and then treated with GS-441524. All 10 cats recovered.
Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis, CA, USA
Gilead Sciences, Foster City, CA, USA
Accepted 14 April 2018

The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies
Feline infectious peritonitis (FIP) is a common and highly lethal coronavirus disease of domestic cats. Recent studies of diseases caused by several R…
Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses
Susan Amirian
Julie K.Levy
Public Health & Healthcare Program, Texas Policy Lab, School of Social Sciences, Rice University, Houston, TX, USA
Maddie's Shelter Medicine Program, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA
Accepted 24 March 2020,

Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses
Recent international epidemics of coronavirus-associated illnesses underscore the urgent medical and public health need for vaccine development and re…
The Atlantic
The background to the long term Cat research GS-441524 and the treatment people are using to cure cats of a deadly coronavirus that kills around 95% of infected cats
Around 5 years ago Gilead sent University researchers 25 or 30 molecules, drawn from the large library of drug candidates that pharmaceutical companies typically maintain.
Two of the molecules worked marvelously in cat cells infected with the FIP virus: GS-441524 and GS-5734, the latter of which is now better known as remdesivir.
After Ebola trials found little benefit, remdesivir became a drug in search of a (human) disease.
Both molecules were effective, so Pedersen decided to pursue the simpler one, GS-441524. He then infected 10 cats with FIP and dosed them with GS-441524. All 10 cats recovered.
“We almost fell out of our chairs,” says Weigner.
in a follow-up field trial of 31 pets with naturally acquired FIP, 25 ultimately made it—an unheard-of recovery rate.
Despite the success, Gilead refused to license GS-441524 for use in cats.
While Pedersen was testing GS-441524 in cats, a different virus—a human virus—was raging halfway around the world in West Africa: Ebola. The virus that causes Ebola is not a coronavirus, but remdesivir is unusually broad-acting for an antiviral, and early results against Ebola were promising. So promising, in fact, that the company was eyeing FDA approval of remdesivir in humans.
According to Pedersen, Gilead worried that the cat research could impede the approval process for remdesivir. Because GS-441524 and remdesivir are so similar, any adverse effects uncovered in cats might have to be reported and investigated to guarantee remdesivir’s safety in humans.
Gilead’s caution about generating unnecessary cat data is standard industry practice. “One of the rules in drug development is never perform a test you don’t have to, if the results could be problematic,” says Richard Sachleben, a retired pharma-industry researcher.
(Gilead declined to comment for this story.)

A Much-Hyped COVID-19 Drug Is Almost Identical to a Black-Market Cat Cure
Cat owners are resorting to China’s underground marketplace to buy antivirals for a feline coronavirus.

In search for COVID-19 treatments, consumer group pushes drugmaker Gilead to test alternative to remdesivir
The pharmaceutical company that makes remdesivir — the only medication that has emergency authorization to fight COVID-19 — should also be conducting human trials on a related drug with…
Pharmalot
The U.S. government contributed research to a Gilead remdesivir patent — but didn’t get credit
By Ed Silverman @Pharmalot
May 8, 2020
September 2015 in which Gilead sought a U.S. patent for a using the compound for any number of coronavirus infections. Although the code Gilead assigned to the compound – GS-5734 – does not appear in the body of the application, experts who have reviewed the chemical structure say the compound is remdesivir.
One month later, some of the same Gilead employees whose names appeared on the patent application were listed as co-authors on a Nature research paper – along with numerous government scientists – showing remdesivir, specifically, held promise in fighting Ebola and other coronaviruses. The paper also noted testing was conducted at high-risk security labs run by the federal government.
The role played by the federal government in developing remdesivir to combat coronaviruses has, in fact, involved various grants to universities, as well as contributions from government personnel at such agencies as the U.S. Army Medical Research Institute of Infectious Diseases, according to Knowledge Ecology International, an advocacy group that first disclosed these connections.
But while it remains unclear the extent to which federal funds contributed to the R&D, the patent is of particular interest because it is tangible evidence that government work yielded something of potential financial value to the company. Yet government employees are not listed as inventors, which one expert suggested should be corrected, especially in an era when federally financed research might be leveraged to collect royalties or, arguably, lower the price of medicines.
“In this case, all of these scientists were really working together very intimately on research that led to the molecule,” said Arti Rai, a Duke University law professor who specializes in intellectual property and a former U.S. Patent and Trademark Office official, who is researching remdesivir patents and the role played by the federal government.
“Gilead actually had a huge number of patents on the molecules, but had to do a tremendous amount of work to figure out which variations of the various molecules would work against the biological models the government had. This patent illustrates the essence of why collaboration between the public and private sectors is important, not just in terms of money, such as grants, but resources.”
“What really matters is how much money the government has put into research, but if their names were on there, it would help to make the case there was a lot of in-kind contribution from the government,” she explained. “Right now, if Gilead tried to assert rights (in response to a patent challenge), there would be no way to know there was some government right to a license to the patent.”
Generally, one must play connect the dots to find government assistance. For instance, the National Institutes of Health gave grants to several universities whose researchers worked with Gilead scientists. Their efforts appeared in a 2018 paper in the American Society for Microbiology, which found remdesivir can combat coronaviruses, noted Tahir Amin, who heads the Initiative for Medicines, Access, and Knowledge, an advocacy group that challenged various Gilead patents on hepatitis C medicines.
We asked Gilead, which several months ago sought to add claims to the patent, for comment and will update you accordingly.
Numerous consumer advocates, academics, and lawmakers have argued that prescription drugs invented with taxpayer dollars should be affordable to Americans. Public Citizen estimated that U.S. taxpayers contributed $70.5 million to remdesivir R&D overall.
“Since we’re paying for the research, we shouldn’t expect to be at disadvantage,” said Jamie Love of Knowledge Ecology International. “The argument that you have to have a high price because a company made big investments is harder to justify when there was a large public financing role in the R&D. A high company may say a high price is necessary, but the rationale falls apart when we’re paying for the R&D.”
Gilead has often been at the center of such debates. As noted previously, the drug maker and the federal government filed dueling lawsuits over patents that formed the basis of a best-selling HIV prevention pill called Truvada. The government claims Gilead should pay royalties on its patents, which stemmed, in part, from taxpayer funding, while the company has argued the government patents are invalid.

The U.S. government contributed research to a Gilead remdesivir patent — but didn't get credit
"This patent illustrates the essence of why collaboration between the public and private sectors is important," said Duke University law professor Arti Rai.
Feb 2020 news article on GS-441524
By Dr. Joette Giovinco
Dr. Joette Giovinco wrote a press article highlighting GS-441524 in Feb 2020 as a promising Covid-19 treatment

Feline coronavirus treatment could stop spread of COVID-19 in humans, doctor says
A drug used to treat a type of coronavirus that only affects cats could be the key to treating COVID-19, the human coronavirus spreading across the world.
Remdesivir is a promising antiviral drug and works by inhibiting the activity of RNA dependent RNA polymerase (RdRp).
GS-441524 is a feline infectious peritonitis virus (FIPV) inhibitor and a predominant metabolite of Remdesivir that is superior to Remdesivir against COVID-19.
First GS-441524 shows comparable efficacy in cell-based models of primary human lung and cat cells infected with the coronavirus. GS-441524 could strongly inhibit feline infectious peritonitis virus (FIPV), with an EC50of 0.78 μM.
Recent research in coronavirus-infected nonhuman primates demonstrated problems with remdesivir that inadvertently showed the antiviral effectiveness of GS-441524. In multiple studies testing remdesivir in coronavirus-infected mice or rhesus macaques, it was rapidly converted to GS-441524 in the bloodstream..... injection of remdesivir in NHP results in GS-441524 being present in serum at concentrations 1000-fold higher than remdesivir throughout a 7-day treatment course (Figure 3b).
Last but not the least, GS-441524 is capable of treating cats suffering from feline infectious peritonitis (FIP) with a 96% cure rate. Importantly, coupled with the robust antiviral activity of GS-441524 against FIP, these data compel further investigations into the therapeutic and prophylactic utility of GS-441524 against SARS-CoV-2.
All in all, GS-441524, a FIPV inhibitor, is a predominant metabolite of Remdesivir and superior to Remdesivir against COVID-19.
GS-441524, a FIPV Inhibitor and the Predominant Metabolite of Remdesivir, is More Effective Against Covid-19 - Immune System Research
GS-441524, a potent FIPV inhibitor, is a predominant metabolite of Remdesivir and superior to Remdesivir against COVID-19.
www.immune-system-research.com

Gilead should ditch remdesivir and focus on its simpler and safer ancestor
Gilead has another antiviral that's easier to make and safer to use than remdesivir. Why isn't it giving that drug any attention? The world can only hope it isn't for the sake of protecting its intellectual property.

Could cat drugs treat humans with COVID-19?
Two experimental drugs for feline coronavirus could show promise against COVID-19.
GILEAD owns patent rights to GS-441524 (2009) and Remdesivir (2017); The 2009 patent of GS-441524 may be invalid due to prior art: as we have seen in the GILEAD vs MERK trial (which Merk initially won), there is a large amount of prior art in the nucleoside space that may invalidate the GS-441524 patent.
Patent Opposition Database: GS-441524 for COVID19 not pursued by GILEAD
The Patent Opposition Database is a site that allows civil society to share resources and learn about the tools needed to oppose the application or granting of unwarranted patents on medicines.
Covid-19 treatnent research drug GC376
A final thought - great research is being done on Covid-19 some of it is below on another drug also tested on cats with FIP. However this drug GC376 was much less successful than GS-441524
The researchers of GC376 are clearly very good and professional
However how is it Covid-19 treatment research can be done on GC376 with cat coronavirus FIP treatment effectiveness being cited as one of the reasons for rapid research, and yet this not happen on GS-441524 when its almost 100% effective against the cat coronavirus FIP and worked well against other coronaviruses plus GS-441524 is potentially better than remdesivir ?
antiviral GC376 out of Kansas State University had previously been tested but only seven out of 20 cats had gone into remission. Those results seemed impressive at the time, but GS-441524 appeared to be even better
"Relapses no longer responsive to treatment occurred in 13 of these 19 cats within 1-7 weeks of initial or repeat treatment(s). Severe neurologic disease occurred in 8/13 cats that failed treatment and five cats had recurrences of abdominal lesions. At the time of writing, seven cats were in disease remission."

Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis - PubMed
Objectives The safety and efficacy of the 3C-like protease inhibitor GC376 was tested on a cohort of client-owned cats with various forms of feline infectious peritonitis (FIP). Methods Twenty cats from 3.3-82 months of age (mean 10.4 months) with various forms of FIP were accepted into a field...
Research on GC376

In a search for COVID-19 treatments, researchers pursue a drug used on cats | SLAC National Accelerator Laboratory
University of Alberta researchers worked with SLAC X-ray scientists to explore the potential of a feline coronavirus drug that may be effective against SARS-CoV-2.
Published: 27 August 2020
Feline coronavirus drug GC376 inhibits the main protease of SARS-CoV-2 and blocks virus replication
GC376 has been used to successfully treat FIP in cats as its breakdown product, GC373, effectively inhibits the Mpro of FCoV. Analogs of these drugs also inhibited the MERS CoV Mpro and blocked viral replication in cells at an EC50 of 0.5 μM14. Our studies show GC376 and GC373 to be effective inhibitors of SARS-CoV-2 Mpro, in agreement with previous studies showing GC373 and GC376 possess broad specificity against the Mpro of coronaviruses and calciviruses (Supplementary Table 2)10,13,14,22. Clearly, these drugs need to be advanced quickly into human trials for COVID-19. SARS-CoV-2 is the cause of COVID-19 and is a virus with a significant mutation rate23. Also, in some patients the virus has persisted longer than 2 months with some possibility of re-infection24. Vaccines are critically important, but still likely a year or more away as this virus may present vaccine challenges.
There are many clinical trials testing drugs re-purposed from their original indications. Remdesivir, a polymerase inhibitor developed as a treatment for Ebola virus25 had initially been used in compassion cases, and is now FDA approved for emergency use in patients with COVID-19......

Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication - Nature Communications
Coronavirus main protease is essential for viral polyprotein processing and replication. Here Vuong et al. report efficient inhibition of SARS-CoV-2 replication by the dipeptide-based protease inhibitor GC376 and its parent GC373, which were originally used to treat feline coronavirus infection.
Last edited: