This is related to the question on why oil and water don't mix. In fact, we could start by asking why water dissolves water. This seems a strange question, but we can then ask why water dissolves things like water.
We are used to seeing water written a H2O, but we can also write this as HOH or H-OH. The OH group is the key to this question as it "likes" water, but it also "likes" other water soluble groups.
We know that sugar dissolves quickly in water.
Looking at the structure of sugar we see
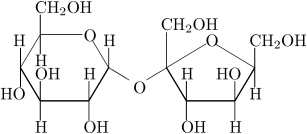
. . . lots of OH groups. So it is easy to understand why it dissolves in water.
So why does table salt dissolve in water. Sometimes we see salt written as NaCl meaning that it is chemically sodium chloride. The symbol Na comes from the Latin word natrium. Salt gets its water solubility in a different way. Again, salt can be written Na+ Cl- because it is formed as "ions" which are electrically charged.
In water salt separates into these electrically charged ions.
Although we have not shown it, water can have a weak charge, so we can write it as H+ OH- and now it is easy to see that the water will "like" the ions and so dissolve them.
We are used to seeing water written a H2O, but we can also write this as HOH or H-OH. The OH group is the key to this question as it "likes" water, but it also "likes" other water soluble groups.
We know that sugar dissolves quickly in water.
Looking at the structure of sugar we see

. . . lots of OH groups. So it is easy to understand why it dissolves in water.
So why does table salt dissolve in water. Sometimes we see salt written as NaCl meaning that it is chemically sodium chloride. The symbol Na comes from the Latin word natrium. Salt gets its water solubility in a different way. Again, salt can be written Na+ Cl- because it is formed as "ions" which are electrically charged.
In water salt separates into these electrically charged ions.
Although we have not shown it, water can have a weak charge, so we can write it as H+ OH- and now it is easy to see that the water will "like" the ions and so dissolve them.
Last edited:

