I was vaccinated against yellow fever a couple of years ago and looked into the subject at the time and again recently.
Vaccines are generally positive but its important for there to be real openness about the risks and whats going on as vaccines are rolled out
There are risks as with all vaccines, that are not much discussed or publicized. A top cancer professor and another person died shortly after having yellow fever vaccinations. Its likely this only got into the news because of the the Professor had links to Prince William.
Prince William pays tribute to Royal Marsden’s Prof Martin Gore, a ‘source of inspiration’

www.theguardian.com
https://www.dailymail.co.uk/health/...ople-DIE-receiving-yellow-fever-vaccines.html
Its not much publicly discussed but the CDC, Dr Fauci and others have quietly highlighted real problems with creating any coronavirus vaccine because of antibody-dependent enhancement (ADE) which is only seen after a vaccinated patient becomes infected with a coronavirus.
Scientific American has stated that "antibodies typical of ADE are present in the blood of some COVID-19 patients"
On Covid-19 it has been said by Dr Fauci
"Scientists will develop a COVID-19 vaccine, but it could end up only being 50 to 60 percent effective, Dr. Anthony Fauci said Friday."
Separately antibody-dependent enhancement (ADE) issues exist with vaccines and were
particularly seen in previous coronavirus vaccines for SARS etc
In some animal trials there were no obvious clinical signs of ADE but post trial work on the animals showed that they had suffered severe health issues
The ADE reaction between the vaccine and the virus has not been obvious in various trials and can be a “serious adverse vaccine event”.
The CDC defines serious adverse vaccine event, as when the "event resulted in permanent disability, hospitalization, life-threatening illness, or death."
Links to Dr Fauci, Scientific American, peer reviewed clinical trials and others explaining some ADE issues are set out below
ADE and Covid-19 American Scientific Article
"Aside from questions of safety that attend any vaccine, there are good reasons to be especially cautious for COVID-19. Some vaccines worsen the consequences of infection rather than protect, a phenomenon called antibody-dependent enhancement (ADE). ADE has been observed in previous attempts to develop coronavirus vaccines.
To add to the concern, antibodies typical of ADE are present in the blood of some COVID-19 patients. Such concerns are real.
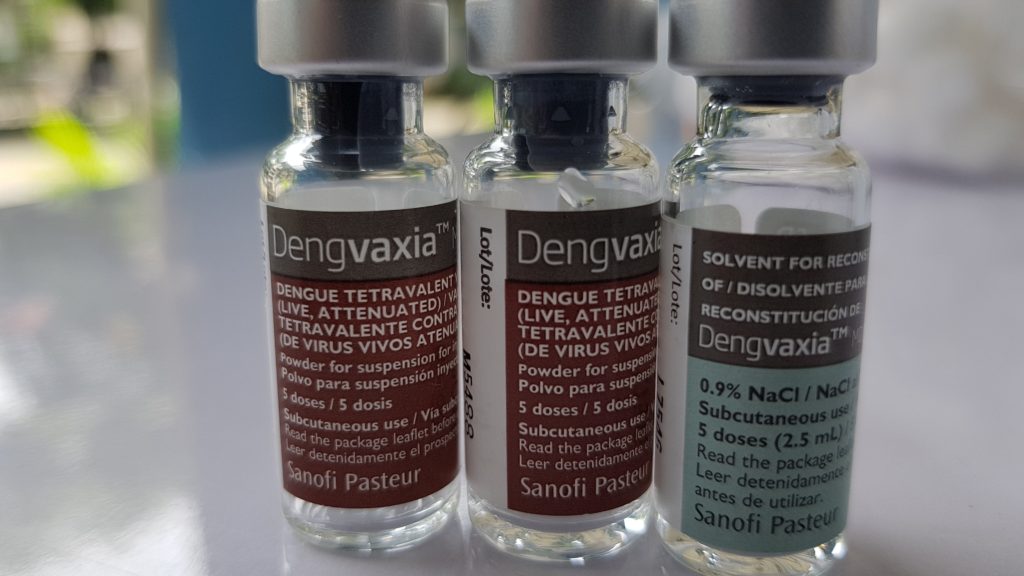
As recently as 2016, Dengavxia, intended to protect children from the dengue virus, increased hospitalizations for children who received the vaccine."
https://www.scientificamerican.com/article/the-risks-of-rushing-a-covid-19-vaccine/
While Dengavxia clearly caused a form of ADE it took a review of 6 years of patient data to determine that there was a problem - and this was done only because of adverse reactions concerns were raised but were initially dismissed by the vaccine maker and still it is not clear who knew what when
"World Health Organization (WHO) revised its recommendations on Dengvaxia, stating that people who have not been infected with the virus should avoid the vaccine"
"There is absolutely no way the Filipino government can claim they didn't know about the potential for ADE-dengue in some vaccinated children," Lanard said. "They only have a claim against Sanofi if they can prove that Sanofi dishonestly reassured them that this worry had no basis."

www.cidrap.umn.edu
"Drug maker Sanofi says that its
dengue vaccine, the world’s first, should only be given to people who have previously been sickened by the virus, according to new long-term data.
In a statement, Sanofi said it had recently examined six years of patient data. Scientists concluded that while the vaccine protects people against further infection if they’ve already been infected with dengue, that’s not the case for people who haven’t previously been sickened by the disease."
“For those not previously infected by dengue virus…the analysis found that in the longer term, more cases of severe disease could occur following vaccination,” Sanofi said. “These findings highlight the complex nature of dengue infection.” [or of an ADE reaction to any infection]
Drug maker Sanofi says that its dengue vaccine, the world's first, should only be given to people who have previously been sickened by the virus.

www.statnews.com
The FDA approved the first vaccine against dengue fever, one that protects against a common disease but has generated significant controversy.

www.statnews.com
ADE in Clinical Trial of Coronavirus Vaccine
In 2004 a study into a coronavirus vaccine trial looked at liver sections of ferrets and showed that all the vaccinated ferrets had severe hepatitis after being exposed to a coronavirus, but displayed no clinical signs of illness. The unvaccinated trial ferrets only had mild hepatitis in their livers – they were injected with a saline solution / another placebo and exposed to the same coronavirus at the same time as the vaccinated ferrets.
https://www.cidrap.umn.edu/news-perspective/2004/12/sars-vaccine-linked-liver-damage-ferret-study
Dr Fauci says about ADE and coronavirus vaccines
"One of the things you have to be careful of when you're dealing with a coronavirus is the possibility of enhancement" Fauci said in an interview with the journal JAMA on April 8. Some vaccines cause a dangerous phenomenon known as antibody dependent enhancement (ADE), which paradoxically leaves the body more vulnerable to severe illness after inoculation.
https://www.livescience.com/coronavirus-covid-19-vaccine-timeline.html
Other researchers have suggested that the severe reactions already seen in Covid-19 could be a form of ADE because of a prior exposure to other coronaviruses, while this is just speculation it seems like it should be researched as part of gaining a better understanding of Covid-19.
https://www.sciencedirect.com/science/article/abs/pii/S1286457920300344
ADE Long Term Vaccine Monitoring issues
Long-term monitoring of adverse vaccine reactions exists, and the CDC has 2 main reporting systems for vaccines – Vaccine Adverse Event Reporting System (VAERS) and Vaccine Safety Datalink (VSD) and will now add V-SAFE for Covid-19
Monitoring of a coronavirus vaccine is not simply a question of looking for any initial ADE reaction to a new vaccine - on going monitoring in the general population is needed generally in case trials do not show longer term consequences. The previous experience of ferrets with a coronavirus vaccine implies some human results may not be known until much later. Separately some researchers have indicated other ADE triggers and different Covid-19 vaccines may use different vaccine adjuvants which could also trigger adverse reactions
The article below outlines the use of a natural adjuvant for Covid-19 Vaccines
The rare Chilean soapbark tree produces compounds that can boost the body’s reaction to vaccines.

www.theatlantic.com
"For all the talk about the cutting-edge vaccines that may just get us out of the COVID-19 mess, little has been written about adjuvants. Perhaps that shouldn’t be surprising: The late Yale professor Charles Janeway famously called adjuvants the “immunologist’s dirty little secret.”
These unheralded helpers can turn a half-baked vaccine into an effective one, or stretch a scarce vaccine supply during a pandemic. Not every vaccine requires an adjuvant, but many do: Of the more than 200 vaccines listed in the Milken Institute’s COVID-19 vaccine tracker, approximately 40 percent are protein-based vaccines, which rarely work without an adjuvant. Yet adjuvants have never attracted much funding from industry and government. “Adjuvants have been the weak link in vaccines for the last hundred years,” says Nikolai Petrovsky, a vaccine researcher at Flinders University in Adelaide, Australia.
Vaccine Adverse Event Reporting System (VAERS) and Vaccine Safety Datalink (VSD)
As we go forward its important that independent researchers can verify what the FDA is telling us about vaccine safety and Vaccine Adverse Events
There does not seem to be much simple reporting available to the public from VAERS about any vaccines. VSD data is regarded as much better but is only open to the CDC and its approved researchers.
There are indications from the CDC and others working with the CDC that VAERS data has quality issues.
A CDC study from 2015 (Tom T. Shimabukuro, MD, MPH, MBA, Immunization Safety Office, Centers for Disease Control and Prevention) states
"Quality and completeness of VAERS reports are variable and many reports lack valid medical diagnoses. The amount of VAERS reporting (30,000 U.S. reports annually) makes it impractical to conduct detailed follow-up on all reports to obtain missing and incomplete information and correct inconsistencies and errors. Because VAERS data do not include an unvaccinated comparison group, it is not possible to calculate and compare rates of adverse events in vaccinated versus unvaccinated individuals and determine if vaccination is associated with an increased risk of an adverse event"
The Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) conduct post-licensure vaccine safety monitoring using the Vaccine Adverse Event Reporting System (VAERS), a spontaneous (or passive) reporting system. ...

www.ncbi.nlm.nih.gov

en.wikipedia.org
CDC itself states that "In 2019, VAERS received over 48,000 reports. About 85-90% of the reports describe mild side effects such as fever, arm soreness, and crying or mild irritability. The remaining reports are classified as serious, which means that the adverse event resulted in permanent disability, hospitalization, life-threatening illness, or death."
https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html
The FDA does not provide VSD information to the public, or provide the data via VAERS (or elsewhere?) related to the vaccinated or vaccinated populations to enable a determination of whether a vaccination is associated with an increased risk of an adverse event.

en.wikipedia.org
Generally vaccines have had positive human health consequences particularly against controlling diseases transmitted from insects etc and things like measles, mumps and rubella, or BCG vaccine etc.
That does not mean they shouldnt be improved on and things like toxins and aluminum adjuvants replaced with natural adjuvant alternatives which are already being used but more expensive.
However the FDA has not been open as to what progress is being made on vaccine safety despite having had a legal duty to report so annually to Congress since 1986.
It is hard not to conclude the the FDA has failed to carry out its duties "to make and assure improvements in the licensing, manufacturing, adverse reaction reporting, research, safety and efficacy testing of vaccines in order to reduce the risk of adverse vaccine reactions"
"Since 1986, HHS has had the primary and virtually sole responsibility to make and assure improvements in the licensing, manufacturing, adverse reaction reporting, research, safety and efficacy testing of vaccines in order to reduce the risk of adverse vaccine reactions. In order to assure HHS meets its vaccine safety obligations, Congress required as part of the 1986 Act that the Secretary of HHS submit a biennial reports to Congress detailing the improvements in vaccine safety made by HHS in the preceding two years."
" ICAN was therefore forced to file a lawsuit to force HHS to either provide copies of its biennial vaccine safety reports to Congress or admit it never filed these reports. The result of the lawsuit is that HHS had to finally and shockingly admit that it never, not even once, submitted a single biennial report to Congress detailing the improvements in vaccine safety. "
It would be helpful to have access to the data on all vaccines for things like the infection and death rates plus the data on vaccine adverse effect rates among vaccinated and unvaccinated populations. A good starting point would be HPV data, the Covid-19 vaccine data when it arrives and then a program to release all vaccine data updates that were required by law since 1986 so that what progress has been made can be clearly seen
There has been limited independent research which has identified vaccine problems
For example a 2017 study funded by the Danish Govt and others concluded that the death rate among children vaccinated with DTP vaccine was up to 5 times higher than all children not vaccinated (and 10 times higher in girls) Other studies have come to similar conclusions
The Introduction of Diphtheria-Tetanus-Pertussis and Oral Polio Vaccine Among Young Infants
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5360569/
"DTP was associated with 5-fold higher mortality than being unvaccinated. No prospective study has shown beneficial survival effects of DTP. "
"It should be of concern that the effect of routine vaccinations on all-cause mortality was not tested in randomized trials."
It would make sense for the whole subject of vaccination to have much greater transparency and at least a total separation of the FDA vaccine regulator from FDA vaccine research and funders
Most serious groups and people who raise question vaccines do not outright object to vaccines, many just want more open data and the removal of things like mercury and aluminum or other adjuvants - basically toxic free vaccines and the use of available natural adjuvant alternatives.
Robert Kennedy Junior who is a prominent leader for vaccine risk awareness, more research and debate, has had all his children vaccinated and has stated he is not against vaccines, but he does want openness. However he has been blocked on social media and the press from responding to critics, google censors searches for his web sites however no one from the pharma side will have an open public debate with him about vaccines.
https://www.nydailynews.com/life-st...speaks-vaccine-requirements-article-1.2160247
Independent views on vaccines are hard to find - the best I could find was Dr. Bernardine Healy MD, former NIH director and former CEO of the American Red Cross she is not an anti vaxxer but has said publicly she believes more research needs to be done into vaccine links to illness - she admits that she only changed her mind after looking at the evidence. In the interview below Dr Healy states that the FDA/government have a formal policy to block research into adverse vaccine effects, which has been set out as policy in writing in reports, and goes back to at least the early 2000s, and Dr Healy says she believes is unscientific.
View: https://m.youtube.com/watch?v=6KFtdSYES6o
An open debate is good for all of us - please can we make this about wanting to find out the truth and not about being right or wrong